Ever since the world registered its first Covid-19 case last year, it has kept the health professionals and scientists around the globe on their toes. With more than 9 million confirmed cases worldwide, the number of active cases is still rising every second. If something that is most awaited in Twenty-twenty is, of course, the Covid-19 vaccine. Given such a large number of populations, wearing a mask and maintaining social distancing would not be of much help. Although we can stop its spread by following corona guidelines, but to completely eradicate the contagion, a vaccine having longer immunity with no major side effect has to be introduced. Globally, more than 100 companies are making covid vaccines, which are currently under different trial phases, among which 11 are already in the final stage of a human trial.
Some of these vaccines are now launched in the market and some of them are either on their 3 phase of a clinical trial or on the verge of getting official clearances, let’s know who is running fast in the race and who lost it before the game got over.
List of Companies Making Covid-19 Vaccines
- Sputnik V by Gamaleya Institute
Named after a satellite launched in 1957, Sputnik V is being developed by Gamaleya Institute in Russia. This vaccine had made headlines a couple of times for its early launch without undergoing any compulsory human clinical trial phase and also when Russian president Vladimir Putin claimed to inoculate the first vaccine to one of his daughters. The vaccine is financed by the head of Russian’s sovereign wealth fund Kirill Dmitriev who also claimed that it is 92% effective and Russia will be the first nation to be Covid free. It is interesting to note that this Russian vaccine will require a second booster shot at a later date.
According to a recent report, Britain’s AstraZeneca (AZN) is now planning to combine its clinical trial with Sputnik V to determine its efficacy together instead of individually.
- Pfizer BioNtech

The Pfizer, an American pharmaceuticals company collaborated with German company BioNtech to develop a messenger RNA based vaccine. The vaccine is supposed to launch before the end of 2020 and has been claimed to be 90% effective against the Covid-19 virus. The vaccine has gone through all three phases of human trials and the US has now given a nod green to its use and will be available in 24 hours as of writing this article. Pfizer is one of the effective Companies making Covid Vaccines.
NOTE: – The US has now become the sixth country to approve the dose of the vaccine, after Saudi Arabia, Bahrain, Canada, Britain, and Mexico.
- Jenner Institute Oxford vaccine Group (AstraZeneca)
Oxford University (OU)has previously registered its name in the list of vaccine development. It has gone through all three phases of the human trial the results of which are expected to come before Christmas. Serum Institute of India has collaborated with Oxford and named its vaccine Covishield (only in India), claims that it will be made available to the front-line workers by the second month of 2020. Although no major side effects have been detected so far, the discussion is going on regarding its logistical and distribution problem, 2-8-degree Celsius is required for its storage, and world cost around 500-600 per dose.
- Moderna
American based biotechnology company Moderna is making covid vaccine and is an mRNA type that has proved to be 95% effective in its trials. Two doses in a gap of 28 days have been found highly effective with no serious side effects. It would be available to the US citizen by the end of twenty-twenty seeking its emergency use.
- Johnson & Johnson
One of the biggest players in the healthcare industry, Johnson & Johnson along with Biomedical Advanced Research and Development Authority, US is making the covid-19 vaccine will be available by the initial month of next year. The 3rd trial phase has already started and even soon its report will be available. Johnson & Johnson is one such company that has promised to offer the vaccine to the US government in its manufacturing cost instead of selling it on profit during the global pandemic.
- GlaxoSmithKline
The GlaxoSmithKline is a known brand in the field of pharmaceuticals. There are several medicines in the market that we are dependent upon, and GlaxoSmith has a large to in it. GSK and another French company Sanofi have collaborated to develop the vaccine. The first and the second trials are completed and the final or third trials are expected to undergo in December.
- Covaccine
Covaaccine is an Indian drug company Bharat Biotech is in the process of making Covid-19 vaccine “Covaccine” in a collaboration with the Indian Institute of virology(NIV). It has completed its all human trial phases and is expected to be available either by the end of 2020 or in the first month of 2021. The vaccine(Covaccine) is different from the rest of the vaccine developed anywhere in the world because it has claimed to develop an antibody from a dead virus to give immunity against the disease.
These are companies making Covid Vaccines, To extend their support to end the global pandemic as soon as possible, several other companies around the world are actively working on their labs to develop a corona vaccine, some of them are CureVac, Inovio, Novavax.
These Companies Stopped Making Covid Vaccines Trail Recently
- Johnson & Johnson
Recently Johnson & Johnson paused its trial for the Covid-19 vaccine due to an unknown illness of a participant involved in the trial phase.
- AstraZeneca
The AstraZeneca too suspended its trial process due to one of the participants falling ill after the shot. AstraZeneca uses the same technology as that of Johnson 7 Johnson.
- V451 Covid-19 vaccine (Australia)
Australia stopped any further development of the Covid-19 vaccine. The reason given was the participants in the early stages of the clinical trial developed antibodies for HIV. These are some companies stopped making Covid Vaccines.
What Are the Stages Involved in the Development of a New Vaccine?
Vaccine development is a long and complex process. It involves several different stages. To launch a new vaccine, it has to go through various trials to determine its effectiveness as well as its side effects. Although the process may vary from institute to institute depending upon them. According to the Centre for Disease Control and Prevention, there are six stages of the development cycle of a vaccine.
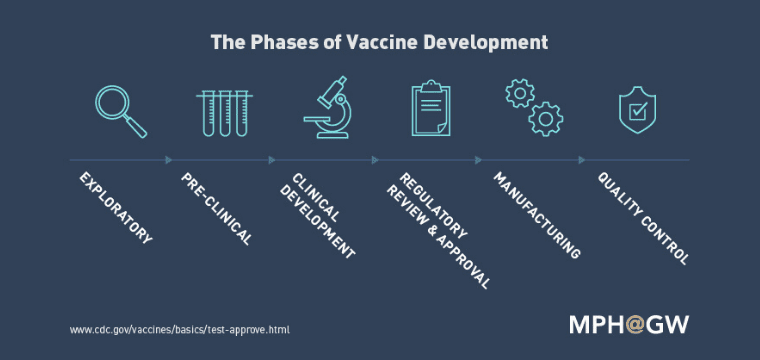
- Explanatory Stage
- Pre- Clinical trial stage
- Review and approval by the regulatory body
- Final Manufacture
- Quality Control
Clinical development is one of the most important processes of vaccine development. It involves three phases, during phase-I, a very small group of individuals are chosen to vaccinate. Then comes the phase-II, where the study is expanded by choosing persons having characteristics similar to those for whom the vaccine is intended. In the thirds and the final stage i.e. Phase III, thousands of people are selected to participate in the human trials to test its efficacy and safety. There are so many vaccine companies who even undergo phase IV depending upon their needs and requirements.
What Is the Approval Process of a Vaccine?
The process of vaccine approval varies from place to place and county to county. As per CDC a new vaccine has to go through these processes before its usage.
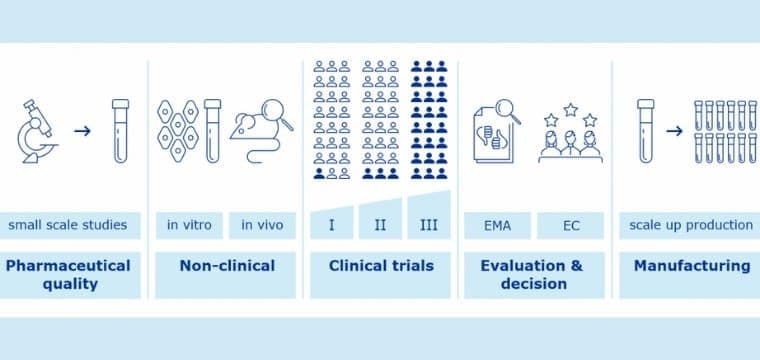
- An application for a new drug investigation
- A pre-license for the clinical trial of a vaccine.
- A Biologics license application (BLA)
- Manufacturing facility inspection
- Presentation of findings to the regulatory body of that country
- Usability testing of product labeling
Read More From Us:

 WhatsApp
WhatsApp